3D Printing Challenges in Healthcare
For additive manufacturing to advance in healthcare, the industry needs better education, standards, and flexible regulation.
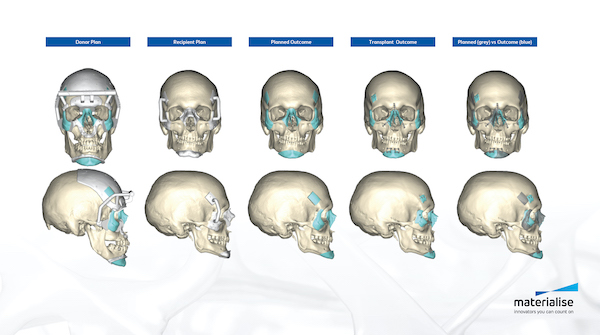
A variety of medical applications benefit from additive manufacturing. Image courtesy of Materialise.
Latest News
November 1, 2022
The next phase of additive manufacturing in the healthcare industry will include increased personalization, new materials, and a more complex regulatory framework.
At the Additive Manufacturing Strategies conference in New York City in early March 2022, experts from across both the 3D printing and healthcare industries participated in panel discussions about the use of additive for medical applications.
Patient-Specific Devices
3D printing is already in use for creating dental and medical implants, and those applications are evolving in a variety of ways. “In ten years we have come a long way in metal implants,” said Oliver Smith, founder of consulting firm Rethink Additive. “We now can create geometries with superior functionality, and implants that can wick cells.”
Benjamin Johnson, vice president, portfolio and regulatory, at 3D Systems, said that the next major step in additive manufacturing will be patient-specific capabilities leveraging patient imaging data that can be converted to 3D models. In turn, those models can be used to print custom-fit implants. “Patient specificity will be the next wave of innovation,” Johnson said. “We will shift from mass production to individual production. Right now, doctors can pick an implant from a product line with a range of sizes; in the future, they can take that patient imaging data and create an implant that exactly fits that patient.”
Phil Reeves, managing director of Reeves Insight, added that new, bio-soluble materials will allow physicians to think differently about permanent and temporary implants. “The body heals itself in a lot of cases, and using permanent implants in a lot of indications does not make sense,” he said. “Historically, we have been limited to inert metals or polymers, because dissolvable materials had a narrow window of mechanical properties.”
Moving forward, chemical advancements could allow for dissolvable implants for specific patients, but that may require changes in the regulatory framework, which is built around approving standardized products.
Micro-printing technologies allow the creation of implants with a 5-micron resolution, which also opens up new possibilities.
Fabric8Labs leverages technologies that allow it to print at the atomic level, using millions of electrodes to build a single layer at one time, said Jeff Herman, company CEO. “We can get finite detail and build 10,000 parts in a single print,” Herman said. “That drives down the cost of the part, and allows us to go after applications that were maybe cost-prohibitive in the past.”
The question of regulatory compliance came up several times. Johnson at 3D Systems said that his company has approached this challenge by having their processes certified, rather than the specific implant or part.
“We think about the worst case scenarios, and how you can bracket those,” Johnson said. “Then you show the data to the regulatory agencies for review, show them the process, show them your envelope, and show them how you ensure safety and effectiveness.”
Herman at Fabric8Labs pointed to one regulatory challenge, in that new materials cannot be approved unless they are used in an existing device, but you cannot get a device to market without an approved material and business case. “That was not a problem with metal because we already had titanium implants. There are no predicates for our new materials.”
There is also an emerging market for point-of-care printing of medical devices and implants, which raises even more regulatory questions. “One aspect we are trying to figure out is, who has control over the hospital as a manufacturer? That is not well-decided yet. How will hospitals set up processes to manufacture medical devices? Over the next several years we will have a better idea of who has jurisdiction, and what types of regulation and guidance they need,” Johnson said.
.png)
Point-of-Care Printing
Hospital-based printing was the topic of an additional panel discussion, which focused on how healthcare providers are using 3D printing to create surgical guides and models, as well as emerging applications around on-site printing.
Albert Woo, chief of pediatric plastic surgery at Rhode Island Hospital and associate professor at the Warren Alpert Medical School of Brown University, has been working with 3D modeling for more than a decade. Another participant, Shannon Walters, is the executive manager for the 3D and Quantitative Imaging Lab in the School of Medicine at Stanford University.
At Stanford, the radiology department has been operating a 3D lab for visualization since 1996. In 2013, they expanded to incorporate 3D printing, and currently use six printers to create several hundred prints each year.
The Mayo Clinic has long been a leader in the use of additive manufacturing. According to Amy Alexander, unit head of biomechanical development and applied computational engineering there, Mayo uses 27 printers inside the hospital for anatomic modeling and surgical guides, and is exploring how to create patient-matched titanium plates on site.
Alexander said that having the print capability on site is key, since the models have to be printed in the hospital. However, obtaining space and funding can be challenging.
“It can be difficult to find that footprint, and barter your way into meetings so that space can be allocated for something that does not have a Category 1 set of codes for billing.” she said. “It takes a champion in radiology and surgery to make the case for why these models are improving surgeries, decreasing time, improving outcomes, reducing the number of revisions, and reducing the number of days in the hospital.” Those indirect outcomes are key for convincing leadership to invest in 3D printing.
She also noted that in some facilities, different departments may set up siloed 3D printing operations, making it difficult to cross collaborate. Because radiology feeds into surgical practices, that department can be a good place to start.
Woo noted that it can also be difficult to get surgeons and doctors engaged in bioengineering because the implants or models being created will not be used until several weeks later. “There are a number of us surgeons who are very interested in the design of implants, but I can tell you from my experience of being the director of a 3D printing lab and talking to other surgeons, the majority of them are not interested in spending time with implants,” Woo said. “They want them to be simply and easy as possible, and they do not look at them until the day of surgery.”
At Mayo, Alexander said that their 3D modeling practice was driven by surgeons, rather than by engineering. However, that required upfront education with the surgical teams to obtain their buy-in. Surgeons and engineers also do not speak using the same terms, so there needs to be a way to effectively communicate among those different specialities.
Woo also noted that many 3D printing companies do not provide seamlessly integrated solutions for 3D printing in a hospital, which requires very specific instructions and processes for sterilization. Printers are also designed to be used by specialists; in hospitals, a variety of users will need to be able to print models and implants, and they may not all have the same degree of engineering and printing knowledge.
Standards for creating 3D-printed anatomic models and guides are still in development. The RSNA 3D Printing Special Interest Group is working on modeling standards. “This legitimizes the field by setting up criteria that are agreed upon by peers in the space,” Alexander said. She also added that the Radiological Society of America and the AMA are trying to create a national registry to determine how much anatomic modeling is actually being used right now.
Regulations in Flux
Emerging regulatory frameworks were also the subject of another panel. According to David Hwang, biomedical engineer for the U.S. FDA, printing does not fundamentally change the risk classification of a medical device. Some changes, however, have been required. The FDA has a process called the De Novo Classification Request for novel medical devices to eliminate the need for a predicate in the approval process.
“Understanding of a new material is a key point,” Hwang said. “Just because we have a new material does not mean a device can be classified as a De Novo device. You can demonstrate that a new metal may perform similarly to another material,”
The FDA did provide guidance on the use of additive manufacturing in 2017, but Hwang pointed out that the science around additive has grown and evolved since then. The FDA has also released a discussion paper around point-of-care printing in healthcare.
“We recognize that it is a hot topic and certainly progressing quickly, and we would like to have guidance completed as soon as possible,” Hwang said. “The paper provides a framework of things that we might be able to accommodate, and what methods people may want to use to go about doing it.”
Because different hospitals may take different approaches (in-house printing versus outsourcing), the guidance will not be one-size-fits all, Hwang said.
According to Steven Kurtz, principal at consulting firm Exponent, the industry needs a well-established set of standards, as well as better training and education.
“If you are going to work in a hospital designing medical devices or models, you need to be exposed to additive within the biomechanical curriculum,” he said.
“Building that workforce will be super important,” Hwang added. “People have issues with printers and copiers just printing a piece of paper. Imagine extrapolating that to a 3D printing, and printing something that is intended to go into a person's body. You need a workforce to manage those systems and make sure they always work properly. ,”
Subscribe to our FREE magazine, FREE email newsletters or both!
Latest News
About the Author
Brian Albright is the editorial director of Digital Engineering. Contact him at de-editors@digitaleng.news.
Follow DE